 |
АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомДругоеЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция
The device, principle of work spectrophotometer. Application spectrophometrical methods of research for definition of concentration of substances in biological liquids
Spectrophotometry - the area of measuring techniques developing methods and devices for definition of spectral characteristics of objects. The analysis of molecular and nuclear spectra absorption has very much great value in medico-biologic researches.
It is probably to define the maintenance of enzymes, hormones, fibers, the vitamins, any inorganic substances in various biological tests by means of spectrophotometer. As probably to lead the analysis of qualitative and quantitative structure of smear of blood. Registration of a degree of easing of monochromatic bunch of light at its passage through substance is a basis of spectrophotometry.
Law of Buger-Lambert-Ber is fair only for a plane-parallel bunch of monochromatic light and at performance of some conditions. Deviations from this law often meet in an expert. Physical and chemical properties of analyzed substance or solution (dissociation, fluorescence, etc.), tool factors (for example: absence of a due degree of monochromaticity of a bunch of light), heterogeneity of studied object in a bunch of light are the reasons of such deviations.
The important principle of spectrophotometry is the principle of optical density according to which the size of optical density of a mix of the connections, submitting to law of Buger-Lambert-Ber and not entering chemical interaction with each other, is equal to the sum of optical density of these connections.
The quantitative analysis of the test containing one substance, includes following operations: 1) registration of a full spectrum of absorption of substance (D the choice of analytical length of a wave, preparation of 6-7 reference (standard) solutions covering all expected range of concentration of defined substance and measurement of optical density of these solutions at analytical length of a wave measure as function of length of a wave. Then build the schedule of dependence of size of optical density from concentration. The most exact results give measurement of optical density in a range 0,05-1,50. The optical density of an analyzed solution is measured concerning a solution of comparison (direct spectrophotometry method). As a solution comparison can be used pure solvent or a solution containing all components of the investigated solution, except for defined substance. Sometimes as a solution of comparison it is possible to use a solution of defined substance of known concentration (usually lower, than in analyzed tests). It is differential spectrophotometry. Spectrophotometry is spent in infra-red, ultra-violet and visible areas of a spectrum.
To the devices working in visible area, concern also spectrophotometer of combinational dispersion which study oscillatory power levels of molecules. They can be used for definition of communications in fibers, numbers of the coupled and not coupled bases in nucleinic acids.
Spectrophotometry of microobjects is independent area of research. Spectra spot photometers are intended for work in visible area of a spectrum. For registration of distribution of absorbing substance on the area of microobject are used a various sort of the scanner: system of optical probes, subject little tables, television (electronic) development of the image. Scanners are supplied with microcomputers, which automatically process the received information and allow to analyze the form of microobjects.
For example, at the automatic analysis of blood smear. Atomic absorptive methods enable definitions of all elements of periodic system and differ sharp selectivity and sensitivity.
Devices for the spectral analysis have electronic devices of processing and management blocks of automatic submission of tests, recorders, blocks of a digital press, the device of automatic development of a spectrum; allow to define some elements on one test.
Spectrophotometry is widely applied in biological researches. It is used for quantitative definition of the diversified biological connections: enzymes, vitamins, hormones, fibers and other nitrogenous substances, nucleinic acids, carbohydrates, spirits, aldehydes, phenols, organic acids, lipids, pigments, of some inorganic substances (for example, sodium, potassium, iron, zinc, chlorine, sulfur.
Devices. Basic units of spectrophotometer are: a source of radiation; monochromator, intended for allocation from a spectrum of radiation of a source of narrow spectral intervals; the receiver of radiation; accounting device. Spectrophotometers are subdivided on one-beam and two-beam, not registering and registering.
One-beam not registering spectrophotometers are widespread in medical laboratories. They represent simple and cheap devices with optical instruments, made from optical glass or quartz. Use of quartz optics enables to spend measurements in the field of 200-1100 nanometers, covering ultra-violet, visible and near infra-red sites of a spectrum. The investigated sample and the standard consistently are located in a light bunch. Results of comparison of factors of a transparency or are specified to optical density on pointer or the digital device.
A hydrogen lamps (for work in the field of a spectrum of 220-320 nanometers) or incandescent lamps (for work in the field of spectrum 320 - 1100 nanometers) are used as the light source of spectrophotometer the ray of light falls on a prism through system of mirrors. This prism decomposes ray of light, forming a spectrum.
In two-beam registering spectrophotometer the stream from a source of radiation is divided into two bunches: the basic and a bunch of comparison (standard). The investigated sample is located in the basic bunch, the standard - in a bunch of comparison. At measurements of factor of a transparency of substances in a solution two identical ditches usually are used, one of which is filled with the investigated sample, and another - the standard.
The light wave is gradually weakened at passage through substance. It occurs in connection with dispersion and absorption of light. Dispersion of light occurs in non-uniform environments provided that the sizes of heterogeneitys are commensurable with length of a wave of light. If heterogeneity of environment is formed by the extraneous particles, randomly distributed in weight of environment, then dispersion of light is named phenomenon of Tindal, and such environments are called muddy, for example a fine fog, a smoke, various suspensions. This phenomenon can be observed, for example, when the narrow bunch of solar beams passes through a dusty atmosphere: light dissipates on motes and all bunch becomes visible at supervision from any party.
The length of a wave of light at dispersion does not change, and intensity of a diffused light that above, than it is less sizes of these heterogeneitys compared with length of a wave. Intensity of dispersion depends also on length of a wave of light. Short waves dissipate much more strongly, than long. Intensity of a diffused light inversely proportional the second degree of length of a wave for large particles, and the third degree - for small particles. Therefore the fine fog has dark blue color, and consisting of larger droplets - white.
Dispersion of light can occur as well in a homogeneous environment on heterogeneitys of substances which are formed at thermal movement of atoms and molecules. For example molecules approach in one points of volume of gas and keep away from each other in other point in pure gas during thermal movement. This kind of dispersion is called molecular dispersion. Intensity of a diffused light inversely proportional the fourth degree of length of a wave of falling light: I ≈ 1/λ4 (Rayleigh law).
In this connection, for example, the glow of a palate is watched blue, and the direct solar radiation gains yellow - red tint, especially at rise and sunset, when this radiation passes more lengthy path in atmosphere. Waves with frequency ωм appear in a diffused light at dispersion of light in homogeneous liquids and crystals. These waves differ from a falling wave with frequency ω0 on the certain size Δ ω. This size depends on molecular structure of the given substance:  .
.
This kind of molecular dispersion is called combinational dispersion of light and matters for studying structure of substance.
At a light scattering the energy saves the electromagnetic nature. At a light absorption she passes in other kinds of an internal energy, thus in material there can be different phenomena: increase of intensity of heal motion (heat effect), excitation both ionization of atoms and molecules. activation of molecules (photochemical effect) etc.
 Energy keeps the electromagnetic nature at dispersion of light. It passes in other kinds of internal energy at absorption of light. The various phenomena can occur in substance thus: increase of intensity of thermal movement (thermal effect), excitation and ionization of atoms and molecules, activation of molecules (photochemical effect). Law of absorption of monochromatic light in a homogeneous environment for a parallel bunch have been established by Buger:
Energy keeps the electromagnetic nature at dispersion of light. It passes in other kinds of internal energy at absorption of light. The various phenomena can occur in substance thus: increase of intensity of thermal movement (thermal effect), excitation and ionization of atoms and molecules, activation of molecules (photochemical effect). Law of absorption of monochromatic light in a homogeneous environment for a parallel bunch have been established by Buger:
The identical part of a stream of energy of a falling light wave is absorbed each layer of the environment of identical thickness, irrespective of its absolute size.
Let's define intensity Id the light wave which have been last a layer of environment by thickness d if the falling wave has intensity I0. For this purpose we shall allocate a layer of environment with thickness dx on distance х from a surface (fig.1,a). Decrease dIx of intensity Ix of a wave owing to absorption of light by this layer under law Buger is proportional to size Ix and thickness of a layer dx: -dIx=  Ixdx
Ixdx
Where  - factor of proportionality. The equation can give a kind
- factor of proportionality. The equation can give a kind 
Deciding this equation, we shall receive 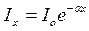 , or for a layer by depth x = d
, or for a layer by depth x = d
 .
.
The graph of change of light intensity Ix depending on depth, layer of medium, which
to drive light, is shown in a fig. 1, b (exponential curve).

 - factor of proportionalityis called a parameter of absorption and characterizes absorptivity of substance. It depends on its nature and a condition, and also from frequency (length of a wave λ0) of light (fig. 2).
- factor of proportionalityis called a parameter of absorption and characterizes absorptivity of substance. It depends on its nature and a condition, and also from frequency (length of a wave λ0) of light (fig. 2).
For metals the parameter of absorption is rather high. It speaks presence in metals free electrons, the compelled fluctuations which are easily raised and have significant amplitude.
The parameter of absorption at dielectric is insignificant, however at them selective absorption of light in the certain intervals of lengths of a wave is observed. The parameter of absorption sharply increases in these intervals. It is connected by that in dielectric is not present free electrons and significant absorption of light occurs only if frequency of a light wave is equal to own frequency of fluctuations of electrons of dielectric. This phenomenon explains line spectrum of absorption of gases in an atomic condition.
Any substance absorbs and reflects electromagnetic radiation. The substances absorbing radiation with lengths of waves 400 - 800 nanometers (a visible range) are painted. Character and size of absorption and reflection of light depend by nature substances and its concentration in a solution. It also is used for the qualitative analysis. The method of the chemical analysis is based on absorption of light by the painted solutions. This method is possible when law Buger-Lambert-Ber is strictly carried out. It is the law of independence of factor of absorption from concentration of analyzed substances in a solution.
If to pass through a solution a bunch of light with intensity I0, that after passage through this layer its intensity will decrease up to I. The attitude T = I/I0 characterizes transmission of light or transparency of environment. Optical density D is size characteristic for the light stream absorbed by substance.
D = l g [ I0/I ] (l)
The optical density can accept any positive values (from 0 up to ∞), but the modern devices allow to measure only D< 2. Change of intensity I of a homogeneous light alter transit it through a layer of substances with thickness d and with concentration С is expressed as follows
 - law Buger-Lambert- Ber
- law Buger-Lambert- Ber
Where k - the factor of absorption (easing) consisting of two components
Factor of absorption ka and factor of dispersion kd
k=ka+kd
C - concentration of investigated substance,
d - thickness of a layer
Sometimes this law enters the name in the form of 
Factors k and k’ are connected among themselves by a parity

Considering (1) it is received 
From here: k = D /Cd
If absorption of light by a solution submits to law Buger-Lambert- Bar the optical density of a solution is directly proportional to concentration of substance in a solution. In this case the graph of dependence of optical density is expressed by the direct line going from the beginning of coordinates. If the parity (1) is not carried out, rectilinear character of dependence is broken. From the law follows:
1. The attitude of intensity of the light stream which has been last through a layer of a solution to intensity of a light stream does not depend on absolute intensity of falling light.
2. Intensity of a light stream decreases in a geometrical progression while thickness of a layer through which passes light, grows in an arithmetic progression.
Quantitative analysis on one impurity in a solution is based on two methods:
1. Method of the calibre graph.
2. Method of comparison.
1. METHOD OF THE CALIBRE GRAPH.
It is necessary to prepare for solutions with known concentration and to measure their optical density. On the received data it is necessary to construct the calibre graph, postponing on axes OY of value of optical density, on 0Х - corresponding values of concentration. Then measure optical density of investigated solutions and under the graph find their concentration. This me thod would serve simultaneously as check of performance of the law Buger-Lambert- Bar.
2. METHOD OF COMPARISON.
It is necessary to measure optical density of a solution with unknown concentration ( )And a standard solution (Dst) at the same thickness of an absorbing layer dx=dst and the same length of a wave. Applying the organic law of absorption of light it is received: Dx=k’Cxdx, Do=k’Codst
)And a standard solution (Dst) at the same thickness of an absorbing layer dx=dst and the same length of a wave. Applying the organic law of absorption of light it is received: Dx=k’Cxdx, Do=k’Codst

WORK OF PHOTOCOLORIMETER
Photocolorimeter electric one-beam it is intended for measurements of factors passing and optical density of the painted solutions in visible area of a spectrum (400 - 630 nanometers). The lamp is used as a light source. The selenic photo cell serves as the receiver of light energy. The device is supplied by system of absorbers - optical filters with maximum transparency 415 nanometers 500 nanometers, 530 nanometers, 600 nanometers (№ 4), 630 nanometers.
Поиск по сайту: