 |
АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомДругоеЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция
Molecular mechanisms of electrochemical potentials of membranes and distribution of a nervous impulse along excitability fibre
|
Читайте также: |
BIOELECTRIC POTENTIALS
All processes of ability to live of organisms are accompanied by occurrence in cells and tissues of electromotive forces. The electric phenomena play a greater role in the major physiological processes: excitation of cells and carrying out of excitation on cells. Biopotentials are a sensitive parameter of various changes in cells in norm and at a pathology. Because biopotentials are directly connected with metabolic processes and a physiological condition of cells.
Distribution of ions defines occurrence of biopotentials. To such potentials concern diffusion, membrane and phase potentials. Diffusion potentials arise on border of section of two liquid environments as a result of various mobility of ions. Ions penetrate on a gradient of concentration. Speeds of diffusion of ions will be defined by their mobility.
Arising Diffusion the potential difference leads to braking of "faster" ions and acceleration of "slower" because forces of an arising electric field are directed against forces of diffusion. Diffusion the potential difference reaches the maximal value during that moment when speeds of diffusion of ions become equal. Diffusion potential difference Е can be determined from equation of Genderson:

Where U - mobility of cation; V - mobility of anion; R - a gas constant; Т - absolute temperature; n - valence of ions; F - number of Faradey; а1 - activity of ions in the field of, whence diffusion goes; а 2 - activity of ions in the field of, where diffusion goes.
Activity of ions is their active concentration. Activity of ions always less than their absolute concentration because ions cooperate with each other. Activity is expressed by product of factor of activity f on absolute concentration С of ions:
a = f ∙C (2)
Diffusion potential will be equal to zero at identical mobility катиона and аниона, and also at absence of a concentration gradient, it follows from the equation (1).
Diffusion the potential can be shown at mechanical damage of cells. Diffusion of ions occurs from a place of damage in the intact sites and diffusion potential arises. Membrane potential is a private kind of diffusion potential. We shall consider diffusion through a semiperable membrane. Diffusion of ions will not be infinite process. After an establishment of balance between forces of diffusion and forces of an electric field on a membrane there is a double electric layer, and diffusion of ions stops. If V=0, the equation (1) turns to Nernst’s equation by means of which it is calculated membrane a potential difference:
 (3)
(3)
As follows from the equation (3), membrane potential depends upon temperature and size of a concentration gradient of ions getting through a membrane.
Potentials of rest, damage and action are membrane potentials by the nature. Phase potentials arise on border of section of two not mixing up substances (for example, a solution of electrolyte in water and any oil). They arise in result of various solubility cations and anions in not water phase. If, cations are dissolved in not water phase better, than anion, they get into it more intensively. Size of phase potentials can be finding from the equation (1).

 electrochemical potential
electrochemical potential
After calculations

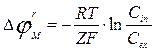
THE THEORY OF THE ORIGIN OF BIOPOTENTIALS.
Membrane theory has been put forward in 1902. However only in 50th years this theory has been developed and experimentally proved by Hodchkin. He has developed the basic ideas about a role of ionic gradients in the mechanism of distribution of ions between a cell and environment. The essence of this theory consists that the potential of rest and potential of action by the nature are membrane potentials. Potentials are defined by semiperable properties of a cellular membrane and non-uniform distribution of ions between a cell and environment.
According to the phase theory of potentials (D.N.Nasonov, V.J.Aleksandrov, A.S.Troshin, 1944), cytoplasm represents a uniform phase not mixing up with water. Ions in cytoplasm are on 80-90% in the connected condition (basically with fibers). Therefore the difference of electric potentials is absent in not raised and intact cells. At excitation or damage of cells ions are released from albumens-ionic complexes of cytoplasm and penetrate in an environment. Potentials of action and damage arise due to diffusion of ions. Polyelectrolyte theory has come in the stead of the phase theory. This theory explains potential of rest and potential of action by properties of molecules of polyelectrolytes in a cell. Polyelectrolyte molecules are molecules with a lot of the fixed charges. Albuminous molecules can be polyelectrolyte molecules. According to this theory, complex polyelectrolyte gel is a basis of cytoplasm. Gel has mesh structure with the fixed negative charges on it. Gel can to accumulate selectively potassium in an ionic kind. At excitation polyelectrolyte structures temporarily lose selectivity to ions that causes in the beginning diffusion of sodium in a cell, and then - potassium from a cell in an environment. Diffusion of ions serves as the reason of occurrence and disappearance of depolarization of cells. Experiences confirm that the potential of rest exists in нативной a cell, and does not result from damage. Presence of polyelectrolytes in cytoplasm is one of the factors, defining presence of some quantity of connected ions калия in a cell.
POTENTIAL OF REST.
Between internal and external surfaces of a cellular membrane always there is a difference of electric potentials. This potential difference measured in a condition of physiological rest of a cell, is called potential of rest. Sizes of potentials of various cells - an intimate fiber of a dog - potential of rest 90 mV, potential of action - 121 mV.
The potential of rest of a cell can be measured by means of the glass microelectrode entered directly in cytoplasm; the second electrode thus is in an extracellular liquid. The thin tip of a microscopic electrode has inside the channel filled by concentrated solution КСl. At introduction of a microelectrode, the membrane of a cell covers its tip, and damages of a membrane do not occur. The basic works on finding-out of mechanisms of occurrence of biopotentials have been executed on large cells of seaweed.
On the basis of a plenty of experiments it has been established, that cytoplasm in a condition of rest of cells always has negative potential in relation to potential of an intercellular liquid. The potential of rest at different cells has size from 50 up to 100 mV.
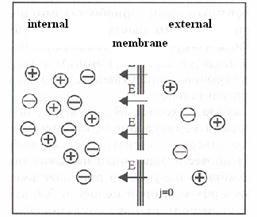
According to modern sights, the reason of occurrence of potentials of cells in rest and at excitation is non-uniform distribution of ions potassium and sodium between contents of cells and environments. Concentration of potassium ions inside of cells at 20-40 times exceeds their maintenance in a liquid surrounding a cell. Concentration of sodium in an intercellular liquid at 10-20 times above, than inside of cells. Such non-uniform distribution of ions is caused by active carry of ions - work sodium – potassium the pump.
Ions of potassium penetrate on a concentration gradient through a cellular membrane into surrounding liquid. Anions can not penetrate through a membrane and remain on its internal party. Ions of potassium have a positive charge, and anions, remaining on an internal surface of membrane - negative. Thus the external surface of a membrane is charged positively, and internal - negatively. Diffusion proceeds up to before moment when balance between forces of an arising electric field and forces of diffusion will established. The potential of rest can be certain under Nernst’s formula:
 (5)
(5)
Where С i and Ce are activity of potassium ions inside and outside of a cell.
For more exact calculation of potential of rest it is necessary to consider not only diffusion of potassium ions, but also diffusion of sodium and chlorine. For more exact definition of potential can be apply generalized equation. This formula was found be Goldman, Hodchkin, Kats and it is called Goldman's equation
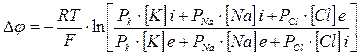 (6)
(6)
Where PK, PNa, Pcl - factors of permeability of a membrane for potassium ions, sodium and chlorine. [K], [Na], [Cl] - their activity inside (i) and outside of (e) cells. The equation (6) allows to define membrane potential not only in a condition of rest, but also at excitation of a cell.
 POTENTIAL ACTION.
POTENTIAL ACTION.
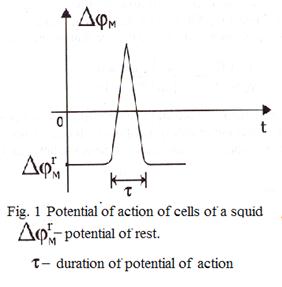
Hodchkin and Huxley (Great Britain) measured potentials of not raised and raised cells of huge squid: it has appeared, that the potential difference was approximately equal to 80 mV (negative) in a condition of rest and the maximal potential difference if equal to + 40mV (positive) at excitation.
Hodchkin and Huxley have formulated a hypothesis that at excitation character of permeability of a membrane for different type of ions varies: it became turns nontight for sodium and, hence, the stream of ions of sodium inside of a cell increases.
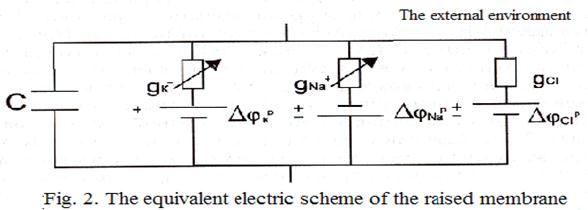
Difference of potentials between surfaces of a membrane rises up to zero, and then becomes positive - comes depolarization of membranes. Further process develops in the opposite direction: the stream of ions of sodium inside of a cell gradually decreases and comes back to the "not raised" value. Such impulse (fig. 1) is called potential of action. Hodchkin and Huxley have constructed also electric model of process of excitation, according to this model the membrane is represented the following scheme (fig.2).
Two variable resistors, the condenser of capacity C, are represented on fig. 2.
 Conductivity
Conductivity 
correspond to two resistors.
3 sources of electromotive power simulating potential differences (calculated on formula Nernst) for ions potassium, sodium and chlorine, and also one more resistance (total resistance of a membrane for ions of chlorine and of some others).
According to this model the full current proceeding through a membrane, is equal to the sum 4 composed: 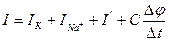
Iк - current of ions potassium;
INa - a current of ions of sodium;
I’- a current of ions of chlorine and other ions, sometimes named by a current of outflow
 - capacitor current.
- capacitor current.
Let's consider a current of potassium. Ions of potassium go through a membrane always, and in both parties. When potential on a membrane (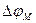 ) is equal to Nernst’s potential, which differently is called potential of balance for potassium
) is equal to Nernst’s potential, which differently is called potential of balance for potassium 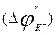 , dynamic balance is kept, i.e. a potassium current
, dynamic balance is kept, i.e. a potassium current 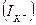 is equal to zero. If the membrane potential deviates from equilibrium value a current of potassium arises, it size can be defined by the Ohm’s law:
is equal to zero. If the membrane potential deviates from equilibrium value a current of potassium arises, it size can be defined by the Ohm’s law: 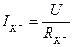 . Having replaced
. Having replaced 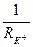 on potassium conductivity
on potassium conductivity 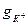 , and U - on size of a deviation membrane potential from equilibrium value, i.e.
, and U - on size of a deviation membrane potential from equilibrium value, i.e. 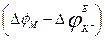 , we receive:
, we receive:
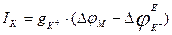
Force of sodium current is defined by the similar formula:
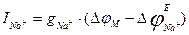 ,
,
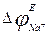 - equilibrium sodium potential, i.e. Nernst’s potential for sodium, which approximately is equal to +40 mV.
- equilibrium sodium potential, i.e. Nernst’s potential for sodium, which approximately is equal to +40 mV.
Forces of currents depend from membrane potential in complex image.
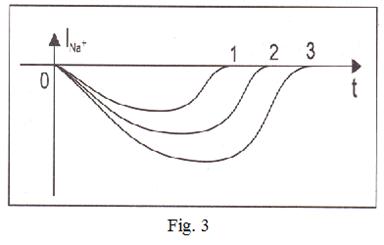
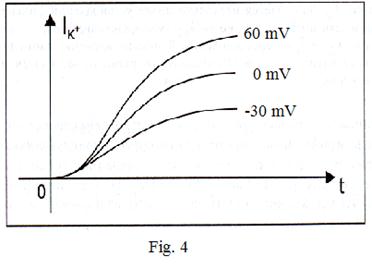
For registration of currents of potassium ions the condition 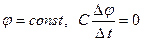 is necessary. Introduction of special blocking substances allows to register these currents. Dependence of a current of ions of sodium on time is shown on fig. 3. Schedules 1, 2 and 3 correspond to various values of the fixed potential. We shall admit
is necessary. Introduction of special blocking substances allows to register these currents. Dependence of a current of ions of sodium on time is shown on fig. 3. Schedules 1, 2 and 3 correspond to various values of the fixed potential. We shall admit 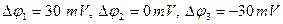
Fig. 4 allows to draw a conclusions:
1. Conductivity g Na depends on time. Because at a constant potential difference a current 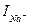 varies in time and it is possible provided that
varies in time and it is possible provided that 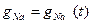 .
.
 2. Under Nernst’s formula equilibrium sodium potential, is equal to nearby + 40 mV. Then in case 3, the fixed value of potential is equal to - 30 mV, a difference
2. Under Nernst’s formula equilibrium sodium potential, is equal to nearby + 40 mV. Then in case 3, the fixed value of potential is equal to - 30 mV, a difference 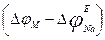 is the greatest (+ 70 мВ) and on absolute size force of a current in this case will be also the greatest.
is the greatest (+ 70 мВ) and on absolute size force of a current in this case will be also the greatest.
3. Conductivity 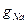 depends upon membrane potential. The similar family of curves for potassium current looks like, shown on fig. 4.
depends upon membrane potential. The similar family of curves for potassium current looks like, shown on fig. 4.
Experiments have confirmed also that the first phase of potential of action is connected with a stream of ions of sodium inside of a cell, and the second - with a stream of potassium ions outside (Fig. 5). In the further Hodchkin and Huxley have offered mathematical model which described changes conductivity and consequently, and currents of ions of sodium and potassium through a membrane during excitation.
One of the basic postulates of this model: in a membrane there are separate channels for carry of ions of sodium and potassium.
The basic properties of channels:
- Selectivity - ability to pass in most cases ions only one type;
- Independence of work of separate channels - a current through this or other channel does not depend upon, whether the current through other channel proceeds or not;
- Discrete character of conductivity of ionic channels: as a first approximation it is possible to approve, that the ionic channel can be in two conditions - opened or closed, transitions between these conditions occur during the casual moments of time and submit to statistical regularities.
 The fixed potential difference on a membrane.
The fixed potential difference on a membrane.
 Dependence of current
Dependence of current  upon time. The arrangement of the schedules in negative area testifies that the current of ions of sodium is directed inside of a cell. On absolute size it first increases, and then again decreases.
upon time. The arrangement of the schedules in negative area testifies that the current of ions of sodium is directed inside of a cell. On absolute size it first increases, and then again decreases.
Dependence of a current  upon time. The arrangement of a curve in positive area testifies that the current of potassium ions is directed outside.
upon time. The arrangement of a curve in positive area testifies that the current of potassium ions is directed outside.
Total current  through a membrane.
through a membrane.
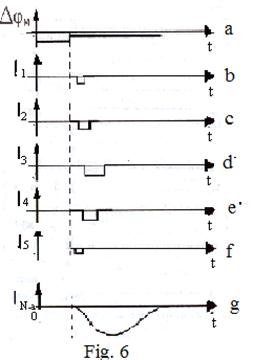
Force of a current through each single channel varies jump, dependence of a total current on time - a smooth curve. It is caused by huge number of simultaneously operating channels (for example, group of cells a squid in length of 1 cm and diameter 1 mm approximately 1010 channels). On fig. 6. communication between currents in single sodium channels and total sodium current is presented.
Dependence of parameters of the channel from membrane potential is shown that after the beginning depolarization of membranes corresponding currents start to change. The selective channel has a sensor control - an element sensitive to action of an electric field. At change membrane potential the value of force acting on it changes. As a result, these parts of the ionic channel moves and changes probability of opening or closing of “a gate”.
Huxley has calculated as varies a membrane potential difference in due course. Results of this calculation with the big accuracy have coincided with the potential of action registered experimentally.
Model Hodchkin and Huxley well reproduce the various phenomena:
- Threshold value depolarized potential;
- If depolarized potential more threshold, then the potential of action develops, it amplitude does not depend upon amplitude of an exciting impulse; if amplitude depolarized potential less threshold, then the potential of action does not arise;
- Presence of the quiet period of a membrane during development of potential of action and the residual phenomena after removal of excitation.
The potential of rest is created as a result of a difference of concentration of potassium ions inside and outside of a cell. However after several potentials of the action, which accompanied by a stream of sodium ions inside on the first phase and potassium ions outside on the second phase, a difference of concentration of these ions inside and outside of a cell should vary. The size of potential of rest should come nearer to zero gradually. Because attitudes of internal and external concentration of potassium ions decreases (in Nernst’s formula). However it does not occur. Probably, there is a mechanism supporting a difference of concentration. By means of such mechanism ions of potassium should to be sucked in inside of a cell (where their concentration and so more), and ions of sodium - to be pumped out outside, where their concentration also is more, than inside. What is the mechanism? Various protein molecules are built in a cellular membrane. It appears that some of them play a role of pumps, sucking in ions of potassium inside, and ions of sodium - outside (an example of active ionic transport). Energy of disintegration of one molecule of ATP has enough for moving 2 ions of potassium and 3 ions of sodium.
Concentration of ions inside and outside of a cell operates work of ionic pumps. Work of the pump is accelerated by surplus of ions of potassium outside of a cell and surplus of ions of sodium inside.
Ion of calcium plays important role in realization of various cellular functions. For muscular reduction many ions of calcium are necessary. Then it is necessary to quickly remove it that the muscle has relaxed. If each ion of calcium acted and left through an external membrane of a cell its fast moving would be impossible. Inside of muscular cells there is a branched out system of cavities and the tubules formed by special internal membrane in which calcium is stored; there it is removed after a relaxation of a muscle. All this membrane "is covered" by calcium pumps. A source of active transport of calcium is also hydrolysis of АТP. We shall consider distribution of potential of action on a nervous fiber. If in some site of excitable membranes the potential of action was generated, the membrane became depolarized, excitation extends on other sites of a membrane.
Let's consider distribution of excitation on an example of transfer of a nervous impulse on axon. Local currents between sites of a surface of a membrane with a more potential (positively charged) and sites with smaller potential (negatively charged) arise in a surrounding solution.
 Local currents are formed and inside axon, and on its external surface. Local electric currents lead to increase of potential of an internal surface of not raised site of a membrane
Local currents are formed and inside axon, and on its external surface. Local electric currents lead to increase of potential of an internal surface of not raised site of a membrane 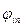 and to downturn of external potential of not raised site of a membrane
and to downturn of external potential of not raised site of a membrane 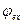 , which settles down near to the raised zone. Thus, negative potential of rest
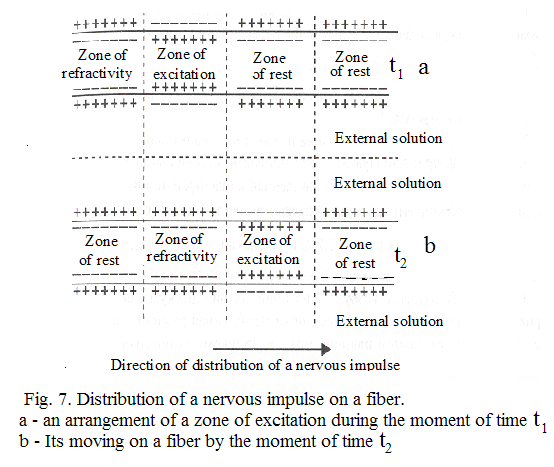
, which settles down near to the raised zone. Thus, negative potential of rest 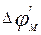 decreases on absolute size, i.e. raises above threshold value. Therefore under action of change membrane potential sodium channels open also the further increase occurs already as a result of a stream of ions through a membrane. Depolarization of membranes occurs, the potential of action develops. Then excitation is transferred further to originally not raised sites of a membrane (fig. 7).
decreases on absolute size, i.e. raises above threshold value. Therefore under action of change membrane potential sodium channels open also the further increase occurs already as a result of a stream of ions through a membrane. Depolarization of membranes occurs, the potential of action develops. Then excitation is transferred further to originally not raised sites of a membrane (fig. 7).
Greater speed of distribution of a nervous impulse on axon of a squid is provided with its huge diameter. Greater speed of transfer of excitation in nervous fibers of vertebrate animals is provided with other ways. Axons of vertebrate animals are supplied by special an environment which increases resistance of a membrane. Excitation on such fiber extends jump from one site, free from this environments, up to another. Nervous impulses are spent on axons to similarly transfer of electric signals on cable - a relay line. The electric impulse is transferred without attenuation because of its strengthening at intermediate relay stations. Sites of excitable membranes play role of intermediate relay stations in axon, where potentials of action are generated.
Поиск по сайту: