 |
АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомДругоеЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция
Spectrum of the brake radiation
1. Spectrum of the brake radiation is continuous (fig. 3)
2. Spectrum has short-wave border
3. Spectrum has  the wavelength corresponding the maximal energy at the given pressure.
the wavelength corresponding the maximal energy at the given pressure.
4. Spectrum has short-wave border 

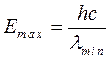
Photons can get this energy at full braking of electrons, having the maximal energy equal to product of a charge of electron on a pressure, e - a charge of electron.

Experimental check of validity of this formula is calculation after it Planck's constant. Value of a constant of Planck, received by this method, most precisely and authentically.
The most typical for radiation is the wave on which it is necessary a maximum of energy of radiation 

The length of a wave depends on energy of photons which defines getting ability of radiation. Therefore getting ability characterize in the length of a wave. More absorbed long-wave radiation is called soft, and less absorbed - short-wave is called rigid. Getting ability of rigid rays is more, than at soft rays. The length of a wave can be adjusted, changing a pressure of a tube. The above the pressure, the is more “rigid” radiation. At change of a pressure changes not only length of a wave, but also the general capacity of radiation. Absorption of x-ray radiation will be carried out more strongly by bone tissues.
 Stream of energy of brake radiation -
Stream of energy of brake radiation - 
U - the pressure enclosed between the cathode and the anode.
I - force of a current in a circuit of a tube.
Z - Nuclear number of substance of the anode (anticathode)
Except for brake, characteristic radiation exists, which has a linear spectrum. Characteristic x-ray radiation is formed at transitions of electrons on internal levels of atoms with a high serial number.
Characteristic radiation is a results of excitation of atoms by electrons with high energy, which get deep into atom and translate close to a nuclear electrons on higher power levels. The quantum is let out at the subsequent transitions removed from nuclear of electrons on a released level.
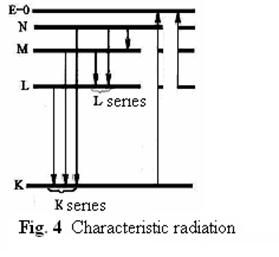
Characteristic radiation arises at transitions of electronоs on internal levels (K, L, M) look fig. 4) atoms with a high serial number. The substance is exposed to strong external influence (bombing by fast electrons or  - particles). Electron of level K is removed from the own orbit and has passed to external level. Electron from any other, higher power level can pass to the released place. Thus a photon is radiated with the frequency corresponding a difference of energy of transition.
- particles). Electron of level K is removed from the own orbit and has passed to external level. Electron from any other, higher power level can pass to the released place. Thus a photon is radiated with the frequency corresponding a difference of energy of transition.
The empty place can arise on internal levels, and transition electronа can occur from any higher level. As a result radiation consisting of separate lines and specific to substance in which it is raised is formed. Lines in a spectrum of characteristic radiation are united in series K, L, M and correspond to transitions of electrons from higher levels on levels K- K-series, L - L-series. The frequencies corresponding lines of these series, are connected with nuclear number of substance in which radiation is raised. English physicist Mozli has established this communication experimentally in 1913 year. 

Where  - frequency of characteristic radiation;
- frequency of characteristic radiation;
R – Ridberg’sconstant;
n - the main quantum number
We can write the Mozli’s law in following kind:
 - frequency of characteristic radiation
- frequency of characteristic radiation
Z - nuclear number of an element, A and B – special constant.
Value  increases for the same size in each series at transition from Z to (Z +1). Therefore elements can be spread out in a number according to increase of nuclear number of substance of the anode. Linear spectrum - the passport of substance. Electrons are raised on the internal levels close to a kernel at excitation characteristic X-radiation. A structure of these level equally at all heavy elements, elements with a high serial number. Therefore characteristic X-ray radiation has a similar appearance at different elements, but frequency of characteristic radiation depends on nuclear number of substance.
increases for the same size in each series at transition from Z to (Z +1). Therefore elements can be spread out in a number according to increase of nuclear number of substance of the anode. Linear spectrum - the passport of substance. Electrons are raised on the internal levels close to a kernel at excitation characteristic X-radiation. A structure of these level equally at all heavy elements, elements with a high serial number. Therefore characteristic X-ray radiation has a similar appearance at different elements, but frequency of characteristic radiation depends on nuclear number of substance.
Поиск по сайту: