 |
АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомДругоеЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция
Research of forces of a superficial tension
Properties of liquids
The substance in a liquid condition keeps the volume, but takes the form of a vessel. If the gravity of a drop is counterbalanced by force of Archimed and only molecular forces act on a drop it takes the form of a sphere. In a condition of weightlessness the liquid accepts the spherical form and outside of a vessel, that has been checked up by cosmonauts. Preservation of volume at a liquid proves, that between its molecules forces of an attraction operate. Hence, distances between molecules of a liquid should be less radius of molecular action. If we shall describe sphere of molecular action around of any molecule of a liquid, then there will be centers of many other molecules inside of this sphere, which will cooperate with this molecule. These forces of interaction keep a molecule of a liquid near its time position of balance approximately during 10-12 - 10-10 sec. Then molecule jumps to new time position of balance approximately on distance of its diameter. Molecules of a liquid between transitions make oscillatory movement near time position of balance. Time between transitions of a molecule from one position in another refers to as time of a settled life. This time depends upon a kind of a liquid and from temperature. At heating a liquid average time of a settled life of molecules decreases. The majority of molecules of a liquid is kept in the positions of balance during time of a settled life (the order 10-11). The small part of molecules passes for this time in new positions of balance.
The liquid possesses fluidity. Time of action of external force of usually many times more time of a relaxation, therefore a liquid flows and takes the form of a vessel in which it is. The ordered arrangement of molecules of a liquid is observed in small volume. The arrangement of molecules of a liquid in great volume appears chaotic. In a liquid there is a near order in an arrangement of molecules and there is no distant order. Such structure of a liquid name quasicrystalline (are similar to a crystal). Identical orientation of molecules on all volume is possible in some liquids with the extended form of molecules. Such liquids refer to as liquid crystals, and their properties differ from usual liquids. At strong heating time of a settled life becomes very small and the near order in a liquid practically disappears. The liquid can find out the mechanical properties inherent in a firm body. If time of action of force for a liquid is not enough, the liquid shows elastic properties. For example, at sharp impact by a stick about a surface of water, the stick can take off from a hand or break; the stone can be thrown so, that it at impact about a surface of water jumps aside from it, and only having made some jumps, the stone will sink in water. If time of influence for a liquid is great, instead of elasticity fluidity of a liquid is shown. For example, the hand gets inside of water easily.
At short-term action of force on a jet of a liquid, it finds out fragility. Durability of a liquid on break is less, than at firm substances, but not so it is a lot of. Compressibility of a liquid is very small, but it is more, than at substances in a firm condition. Breaks inside of a liquid in which there are no extraneous substances, for example air, can be only at intensive influence on a liquid, for example at distribution to liquids of ultrasonic waves. Such emptiness inside of a liquid cannot long exist and sharply slam, i.e. disappear. This phenomenon is named cavitation (from Greek – a cavity).
The molecules which are being depth of a liquid, are surrounded by molecules of a liquid from different directions and their actions on these molecules are mutually counterbalanced. Interaction of the molecules which are being a superficial layer of a liquid, with molecules pair or air above a liquid weak. It can be neglected. It gives to a superficial layer of a liquid (the thickness equal to radius of sphere of molecular interaction) specific features. On each molecule in a superficial layer from molecules of a liquid surrounding it forces in two directions operate: lengthways (on a tangent) and perpendicularly (normally) to a surface of a liquid. Normal components, developing among themselves, lead to occurrence of the force, directed to perpendicularly superficial layer, in depth of a liquid. Pressure of molecules of a superficial layer on next, located below, the layer of molecules of a liquid has received the name of molecular pressure. Forces of molecular pressure operate only on molecules of the liquid and do not act on a body shipped in a liquid. Tangents components of molecular forces are directed along a superficial layer, they pull together molecules of a superficial layer, and the superficial layer appears in a condition of a pressure. Such forces have received the name of force of a superficial tension.
The size measured by force of a superficial tension, the area of a surface of a liquid acting on everyone unit, is called factor of a superficial tension.
The molecules of a liquid which are being on border with a firm body, cooperate both with molecules of a liquid, and with particles of firm substance. If force of an attraction from particles of a firm body there are more than forces of an attraction between molecules of the liquid molecules of a liquid stick to a firm body, there is a wetting a surface of a firm body by a liquid. At pushing away of molecules of a liquid from a surface of a firm body occurs nonwetting. The bent surface of a liquid refers to as a meniscus. At a moistening liquid the concave meniscus, and at nonwetting - convex is formed.
Forces of a superficial tension create additional pressure under a meniscus, therefore the general pressure near the bent surface will be more or less, than above a horizontal surface.
At small radiuses of a meniscus, for example in pipes of very small section - capillaries, superfluous pressure under a meniscus can be reach significant. Such phenomenon occurs in an organism at gas embolism - corking of a blood vessel of bubble of gas. Gas bubbles in blood can appear at divers at fast rise from the big depth on a surface, at pilots and cosmonauts at Infringement of hermetic sealing of cabins or a survival suit at the big height. It is caused by transition of gases of blood from the dissolved condition in free – creation of gases as a result of downturn of surrounding atmospheric pressure. The leading part in formation gas bubbles at reduction of pressure belongs to nitrogen as it causes the basic part of the general pressure of gases in blood and does not participate in gas exchange of an organism and an environment.
The phenomenon of a raising or lowering of a level of a liquid in narrow pipes in connection with action of additional pressure refers to capillary property.
Capillary properties porous bodies, for example a filtering paper, dry chalk, friable ground, etc. the Capillary phenomena are of great importance for a life of plants as promote a raising of water and nutritious solutions from ground along a trunk of a plant.
Superficial tension.
Characteristic property of liquids is the superficial tension formed on a free surface of a liquid i.e. on border with the gaseous environment. This phenomenon is connected by that on a molecule and, taking place on a free surface (fig. 1), forces of an attraction on the side of environmental molecules of a liquid operate much more strongly, than from the side vapors of liquids or gas on which the liquid borders.
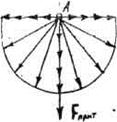

Fig. 1 Fig. 2
If conditionally to choose on a surface of a liquid a piece in length ℓ (fig. 1) forces of a superficial tension can be represented pointers perpendicular to a piece.
The value δ is called superficial tension. It is measured the relation of force of a superficial tension to length of a piece on which force operates.
δ=F/ℓ
The superficial tension can be certain as work which is necessary for making against force of a superficial tension
δ=A/S
The superficial tension of biological liquids can serve in some cases can serve as the diagnostic factor. So, for example, at disease by jaundice a superficial tension wet sharply decreases owing to occurrence to urine of bilious acids. At diabetes and some other diseases the contents of lipase in blood raises. The contents of lipase judge on change of a superficial tension of a solution of threebutilen at addition in it of blood. For definition of a superficial tension in medical practice use a method of falling drops. At the slow expiration of a liquid the drop is formed of an aperture or from a vertical tube. At the moment of tearing of drops force F =2πrδ superficial a tension it is equal to a gravity of drops Q=mg=ρυg, 2πrδ= ρgυ (r -radius of neck of drops, ρ is density of liquids, V -volume of a drop)
δ= ρVg/2π (l)
To measure radius of neck practically it is impossible (it it is possible to make at photographing a drop at the tearing moment), therefore using a method of falling drops, resort to a comparative way. If the superficial tension is known of standard liquid δ0, for example waters, then formula (1) can be written down.
δ0=ρ0 V0 g /2π (2)
If to take identical volumes of water and a researched liquid V and having counted up quantity of drops n in these volumes, then it is possible to calculate volume of a drop.
V0= V1/n0 (waters), V= V1/n (a researched liquid)
Having substituted these expressions accordingly in the formula (1) and (2) and having taken their relation, we shall receive
δ/ δ0= ρn0/ ρ0n (3)
δ= δ0 ρn0/ ρ0n (4)
The factor of a superficial tension depends upon nature liquids and on temperature (decreases at its increase), but does not depend upon size or the form of a surface. Unit of measurement of superficial tension is N/ m or dyne/cm.
Let's result values of a superficial tension for some liquids at temperature
Table 1
| Liquid | δ (N/m) | Liquid | δ (N/m) |
| Water | 0,0725 | Mercury | 0,470 |
| Bile | 0,0480 | Whey of blood | 0,060 |
| Milk | 0,0500 | Ether | 0,0170 |
| Urine | 0,0660 | Spirit | 0,022 |
Density of some water solutions (kg/m3) at 20˚С different concentration
Table 2
Поиск по сайту: