 |
АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомДругоеЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция
Ultrastrucrure and function of function of biological membrane
Membranes have huge value in creation of structure of cells. Membranes surround all cytoplasm and limit it from an environment. Penetration of substances into a cell and from a cell depends on properties of a membrane.
Properties and a chemical compound of a membrane are often studied on shells of erythrocytes. In 1925 Gorter and Grendel spent experiences with lipids, extracted from membranes of erythrocytes. They have found out, that the area of the monolayer borrowed lipids, twice more than the total area of a surface of all erythrocytes. The conclusion has been made, that lipids of membranes are located in the form of a molecular layer. However many data testified also to presence in a cellular membrane of protein molecules. Presence in membrane fibrillar proteins explains properties of membranes - an extensibility, elasticity and ability of some of them to reduction. The phospholipids two-layermolecular layer makes a structural basis of any biological membrane. It carries out function of a barrier for ions, molecules soluble in water and function of a basis, and also a matrix for membrane enzymes, receptors and other proteins built in membranes, glycolipid and glycoprotein. The second model of a membrane - flat two-layer phospholipids membranes. The third modeling system is liposome. Penetration of substances into a cell and from a cell depends upon properties of membranes.
Membranes possess extensibility, elasticity, ability to reduction, a low superficial tension, good permeability. Membranes create borders of section between various sites, phases in which biochemical transformations, biophysical processes precede.
Membranes coordinate and adjust these processes. Cellular membranes posses the important role in maintenance of adhesion-coupling of cells with each other, causing existence of a tissue.
Functions of biological membranes:
- Mechanical - membranes create section between a cell and environment surrounding it, providing independent, complete functioning of a cell;
- Barrier - maintenance of a selective, adjustable passive and active exchange between substance of a cell and an environment;
- Matrix - maintenance of the certain positioning of protein-enzymes concerning substrata.
Besides on internal membranes of mitochondrion there is synthesis АТP; on membranes there is a generation of biopotentials, with participation of membranes occurs mechanical, acoustic, olfactory, flavoring, visual reaction. The basic components of membranes are proteins and lipids (on a share of carbohydrates can have about 10 % of weight of membranes).
Phospholipids molecules form basis (matrix) of a membrane, every molecule of which has a polar head and non polar tail (fig.4).
 Fig. 4. A molecule of water (at the left) and a molecule of phospholipids (on the right), consisting of a polar head and non polar tail.
Fig. 4. A molecule of water (at the left) and a molecule of phospholipids (on the right), consisting of a polar head and non polar tail.
Heads of phospholipids molecules well contact to polar molecules of water, the tails consist of fat acids. In a water solution a molecule of phospholipids spontaneously gather and form a double layer, thus heads settle down on the surfaces turned to water, and waterproof tails are directed to each other and supersede from internal area of a molecule of water. Thickness of lipid double layer makes approximately 4-5 nanometers (fig. 5).
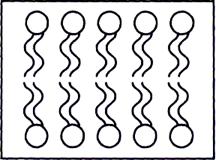
Fig. 5. Phospholipids double layer.

Fig. 6. The protein penetrating phospholipids double layer. 1 integrated protein; 2 peripheral proteins.
The membrane consists not only of molecules of phospholipids, but from molecules of protein (fig. 6). Membrane proteins are divided usually into 2 kinds: external (peripheral) and internal (integrated) (see fig. 6). The general thickness of a membrane is equal about 8-9 nanometers.
On structure the membrane concerns to liquid crystals. But on surfaces of double layers there is an intensive thermal movement. It is called lateral diffusion. It can be presented as sequence of transitions of molecules from one position to another with frequency approximately 107 - 108 in a second (approximately for this time the molecule bends around all surface of a membrane). Much less often molecules jump from one surface of membrane on other. For phospholipids this process is called «flip-flop» and there is 1 time within several hours.
TRANSPORT OF SUBSTANCES THROUGH BIOMEMBRANES.
Moving of substances to a cell or from it in an environment is carried out by two ways by means of passive and active transport.
Passive transport is always carried out due to the energy concentrated in any gradient, and energy of metabolic processes of cells (energy of hydrolysis АТP (adenosinetriphosphat)) is direct on this carry is not spent.
Passive transport always occurs in a direction of gradients, from higher power level to lower.
The basic equation for passive transport is equation of Terrell:
 (1),
(1),
where j - the density of a stream of particles, is equal to quantity of the substance which are passing for a time unit through an individual platform; u - mobility of particles; C - concentration;  - gradient of electrochemical potential, i.e. the size showing speed of its change with distance х. Set of concentration and electric gradients is called an electrochemical gradient. Sign the minus in the right part of the equation shows decrease of function
- gradient of electrochemical potential, i.e. the size showing speed of its change with distance х. Set of concentration and electric gradients is called an electrochemical gradient. Sign the minus in the right part of the equation shows decrease of function  .
.

Fig. 7. Dependence of electrochemical potential  from coordinate х (it is resulted for a substantiation of equation of Terrell).
from coordinate х (it is resulted for a substantiation of equation of Terrell).
Let's substitute expression for electrochemical potential of the diluted solution in equation of Terrell:
 (3)
(3)
Removing the brackets, we shall receive the equation the Nernst-Plank:
 (4)
(4)
The physical sense of the formula (4) consists that the size and a direction of density of a stream of particles j is defined by two factors: a gradient of concentration  and a gradient of electric potential
and a gradient of electric potential  . If both of a gradient are equal to zero it is equal to zero and density of a stream of particles.
. If both of a gradient are equal to zero it is equal to zero and density of a stream of particles.
If gradients have the same sign, then the density of a stream of substance will be directed from areas with great value of both parameters (electric potential and concentration) in the area of with their smaller value - along axis X. If gradients have a different sign the direction of density of a stream depends on what from composed is more on absolute size. If both composed are opposite on a sign and equal each other on the module, in this case the density of a stream is equal to zero.
If in transport process participate no electrolyte (charge Z=0) or the gradient of potential is absents  , then Terrell’s equation passes in Fick's equation
, then Terrell’s equation passes in Fick's equation
 , (5)
, (5)
where D - the coefficient of diffusion is equal to product uRT. In this case it is possible to tell, that passive transport is directed aside reduction of concentration.
Поиск по сайту: